

Hilltop Group pioneers advancements in longevity therapies through its state-of-the-art Phase I Clinical Research Unit. Specializing in Advanced Therapy Medicinal Products (ATMP), we provide comprehensive services including Phase I trials, GMP cell processing, and cutting-edge biomarker platforms.


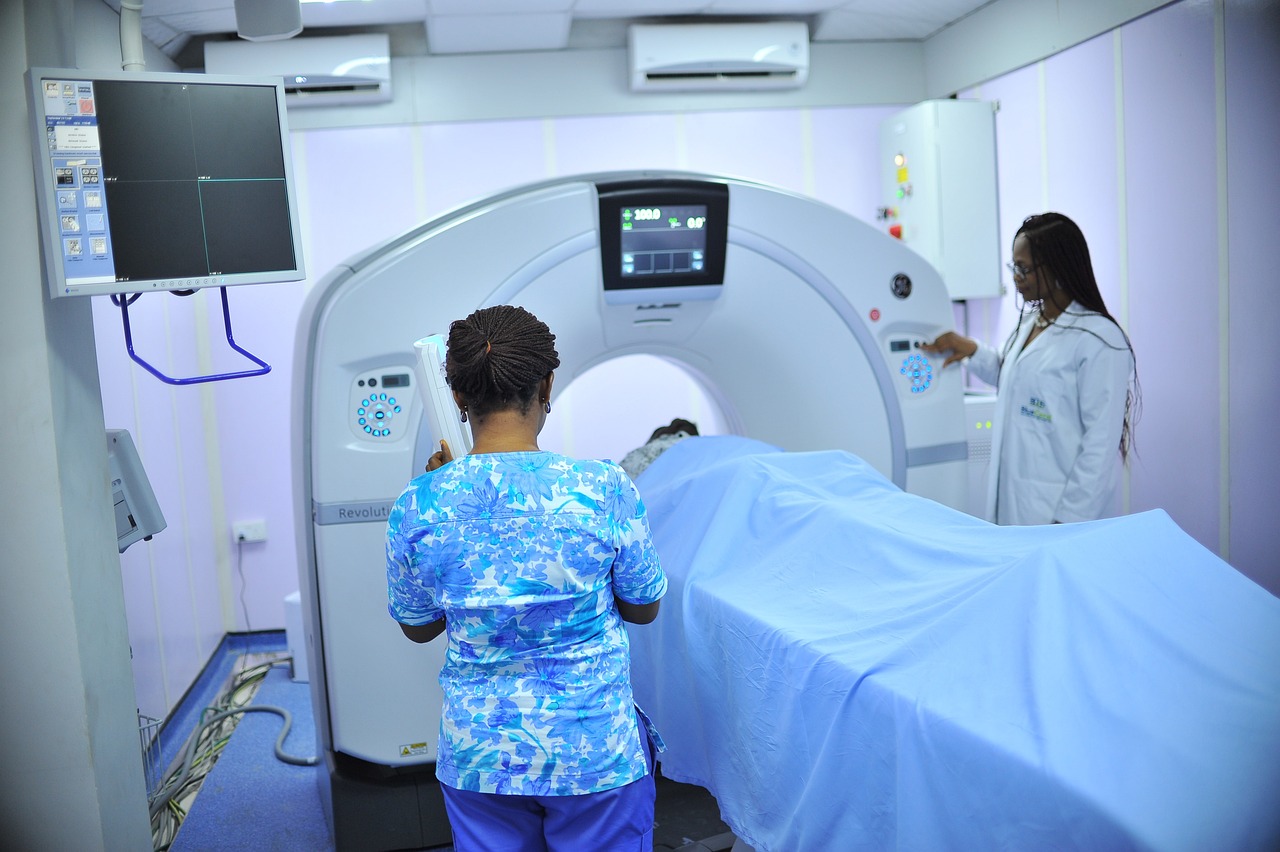

Comprehensive late-stage development to validate efficacy, demonstrate safety, and generate real‑world evidence for ATMPs and longevity therapeutics.

At Hilltop Group, we are pioneers in Experimental Therapies, pushing the boundaries of traditional medicine. Our commitment to innovation allows us to explore uncharted territories in longevity treatments, providing patients with access to groundbreaking therapies. Each experimental protocol is crafted with precision, ensuring compliance with regulatory standards while prioritizing patient safety.
By collaborating with biotech startups, we foster a vibrant ecosystem that encourages the development of novel solutions. Our goal is to transform the landscape of healthcare, offering hope and new possibilities for those seeking advanced treatment options. Discover the future of medicine with us.


Our integrated approach aligns research capabilities with regulatory compliance and commercial growth—so your program can move faster with confidence.
Fill out the form and we'll respond within 24 hours.
Alina Paster — Business Development
Email: bd@hilltopgroups.com
Phone: +1 917 329 22 52
Montana, USA